Mrna Vaccine Safety In Animals
The post appears to be a screenshot of an online article which makes a number of incorrect claims. As the race to authorize the use of COVID-19 vaccines continues posts online are spreading misinformation.

A Single Immunization With Nucleoside Modified Mrna Vaccines Elicits Strong Cellular And Humoral Immune Responses Against Sars Cov 2 In Mice Sciencedirect
Severe systemic adverse events were reported by 5 to 10 percent of trial subjects.
Mrna vaccine safety in animals. Animal studies contributed to the scientific understanding of how these new types of mRNA vaccines work. Could mRNA COVID-19 vaccines be dangerous in the long-term. The safe dose was too weak and repeat injections of a dose strong enough to be effective had troubling effects on the liver in animal studies.
A viral Facebook post claims that all animals involved in Covid-19 vaccine studies died months later from immune disorders sepsis andor cardiac failure. MRNA vaccines have elicited potent immunity against infectious disease targets in animal models of influenza virus Zika virus rabies virus and others especially in recent years using. Anyone whos over 70 who gets one of these COVID-19 mRNA vaccines will probably be sadly die within about two to three years.
BioNTech is a German company established to work on immunotherapies in 2008 by a Turkish couple who. Moderna was not the only one working on an mRNA approach to vaccines. While an mRNA vaccine has never been on the market anywhere in the world mRNA vaccines have been tested in humans before for at least four infectious diseases.
In animal studies after mRNA injections have been administered to cats when the virus arrived once again into the body it arrived like a Trojan Horse undetected by the cats own immune system. Twenty years after the demonstration that messenger RNA mRNA was expressed and immunogenic upon direct injection in mice the first successful proof-of-concept of specific protection against viral infection in small and large animals was reported. Lee Merritt explains that mRNA technology is not a vaccine mirroring what Dr.
The study this particular claim is based on was about severe acute respiratory syndrome SARS and published in 2012. There is a race to get the public vaccinated so we are willing to take more risk. Rabies influenza cytomegalovirus.
Pfizer and Moderna did not skip animal trials when testing their COVID-19 vaccines. Moderna was founded in 2010 to produce vaccines based on the new mRNA technology and the company had been growing as a vaccine manufacturer when the COVID-19 virus appeared. However their application has until recently been restricted by the instability and inefficient in vivodelivery of mRNA.
For example when the 2016 Zika virus outbreak occurred researchers developed a nucleic acid vaccine that protected against Zika virus infection in mice and nonhuman primates. The Pfizer and Moderna vaccines are mRNAs vaccines that skipped animal trials because using mRNA vaccines on animals triggers dangerous inflammation. Animals vaccinated with 10 or 100 g of mRNA-1273 had reciprocal ID50GMTs of 501 and 3481 respectively values that are 12 times and 84 times as high respectively as in human convalescent-phase serum Figure 1F.
David Martin also stated recently. Systemic adverse events such as fatigue muscle aches headache and chills are common. In the following interview Dr.
Animals vaccinated in the 2012 study referred to in this video did not die when exposed to a live virus and the research did not involve mRNA. One argument supporting the long-term safety of mRNA vaccines is that mRNA genetic material is incredibly fragile to the point where such vaccines have a strict storage condition at -20 to. MRNA vaccines represent a promising alternative to conventional vaccine approaches because of their high potency capacity for rapid development and potential for low-cost manufacture and safe administration.
The only evidence on safety of mRNA vaccines comes from small phase I and phase II trials of SARS-CoV-2 vaccines with follow-up typically less than two months.

Prospects For A Safe Covid 19 Vaccine Science Translational Medicine

Covid 19 Mrna Vaccines How Could Anything Developed This Quickly Be Safe News Uab

Mrna Vaccine With Antigen Specific Checkpoint Blockade Induces An Enhanced Immune Response Against Established Melanoma Molecular Therapy

Coronavirus Vaccine Clinical Trial Starting Without Usual Animal Data Stat
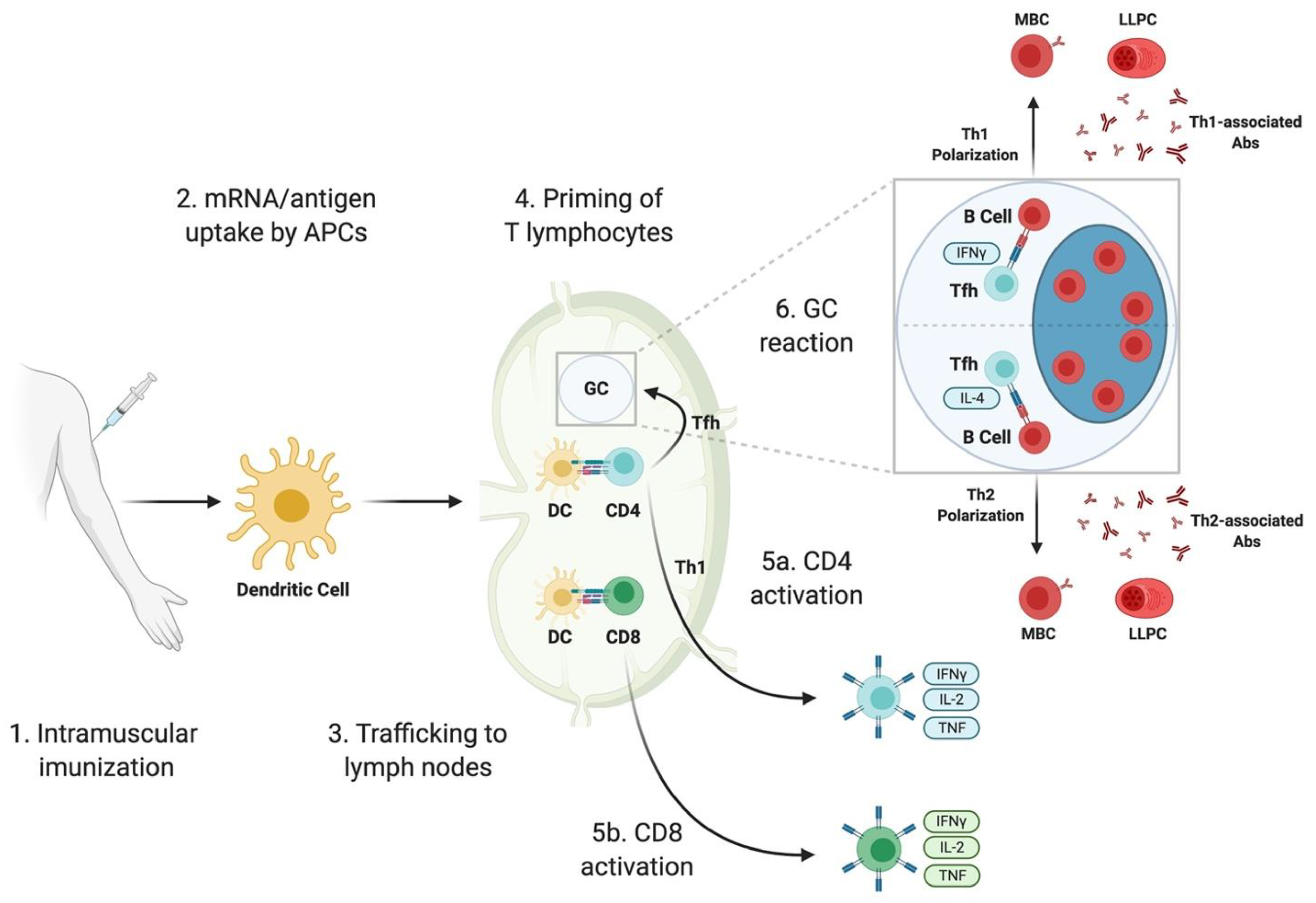
Vaccines Free Full Text Sars Cov 2 Mrna Vaccines Immunological Mechanism And Beyond Html

Covid 19 Vaccines A Race Against Time In The Middle Of Death And Devastation Journal Of Clinical And Experimental Hepatology

A Multi Targeting Nucleoside Modified Mrna Influenza Virus Vaccine Provides Broad Protection In Mice Molecular Therapy

Mrna Vaccines Faq Infectious Diseases And Vaccinations Thl
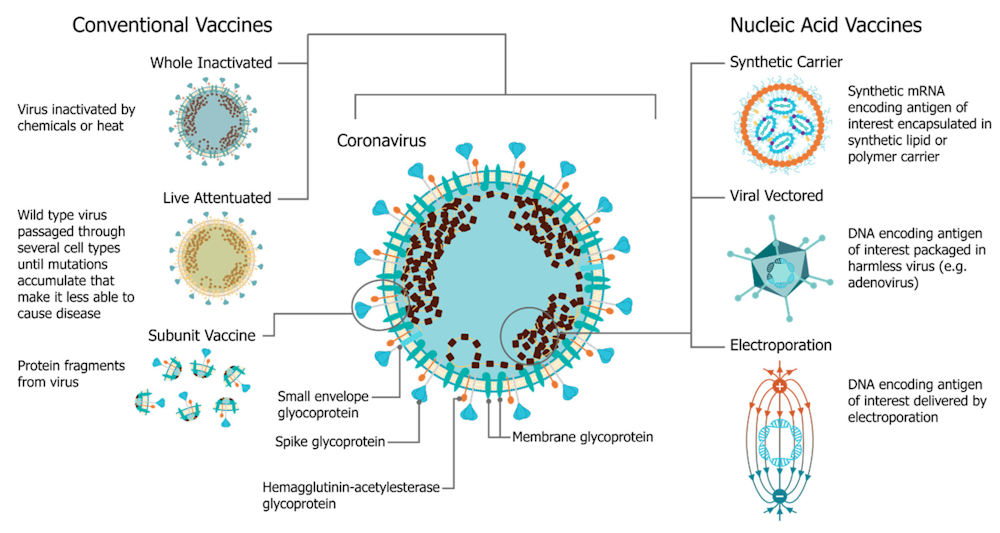
A Mix And Match Approach To Covid 19 Vaccines Could Provide Logistical And Immunological Benefits Pbs Newshour
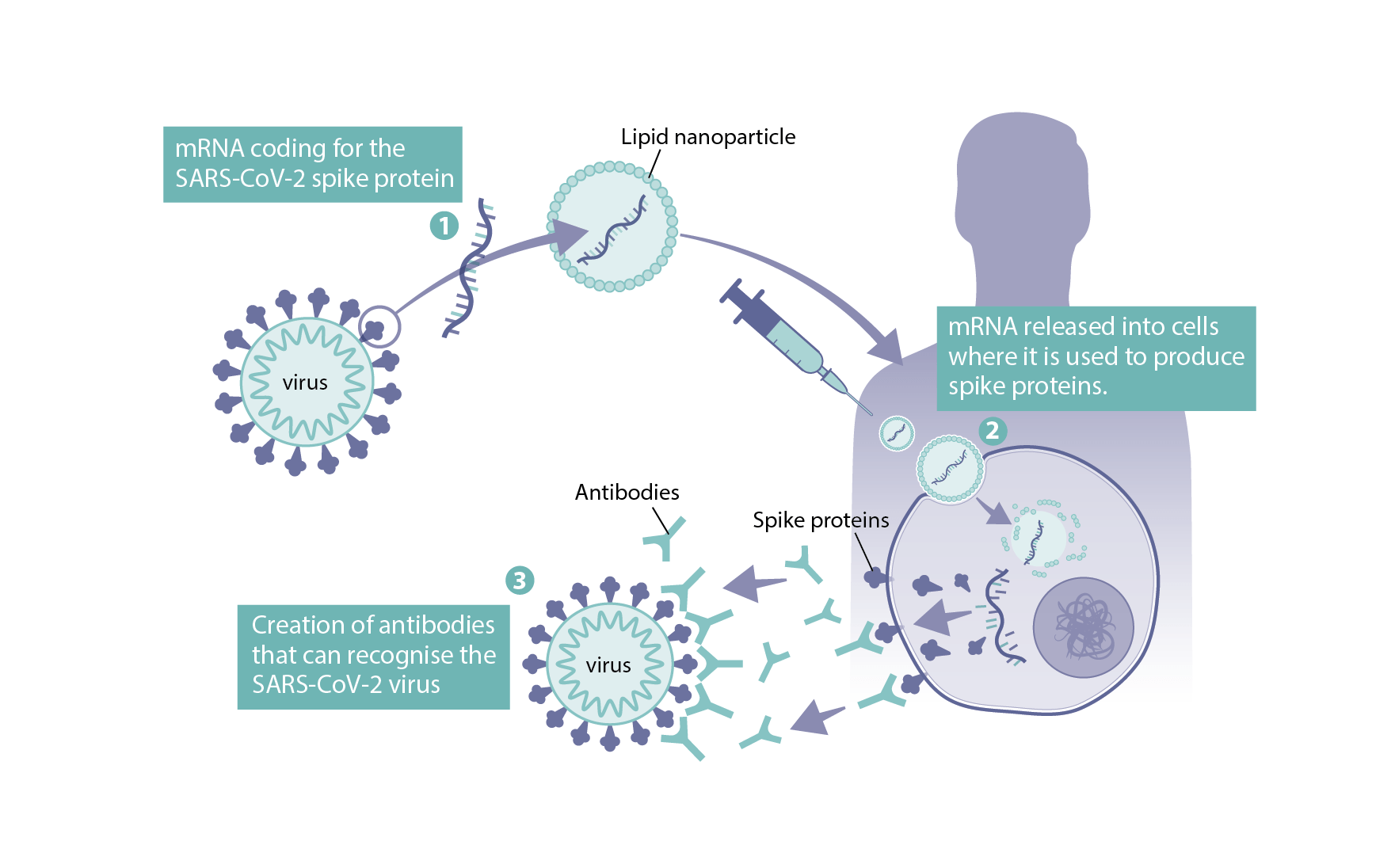
How Were Mrna Vaccines Developed For Covid 19 Health Feedback
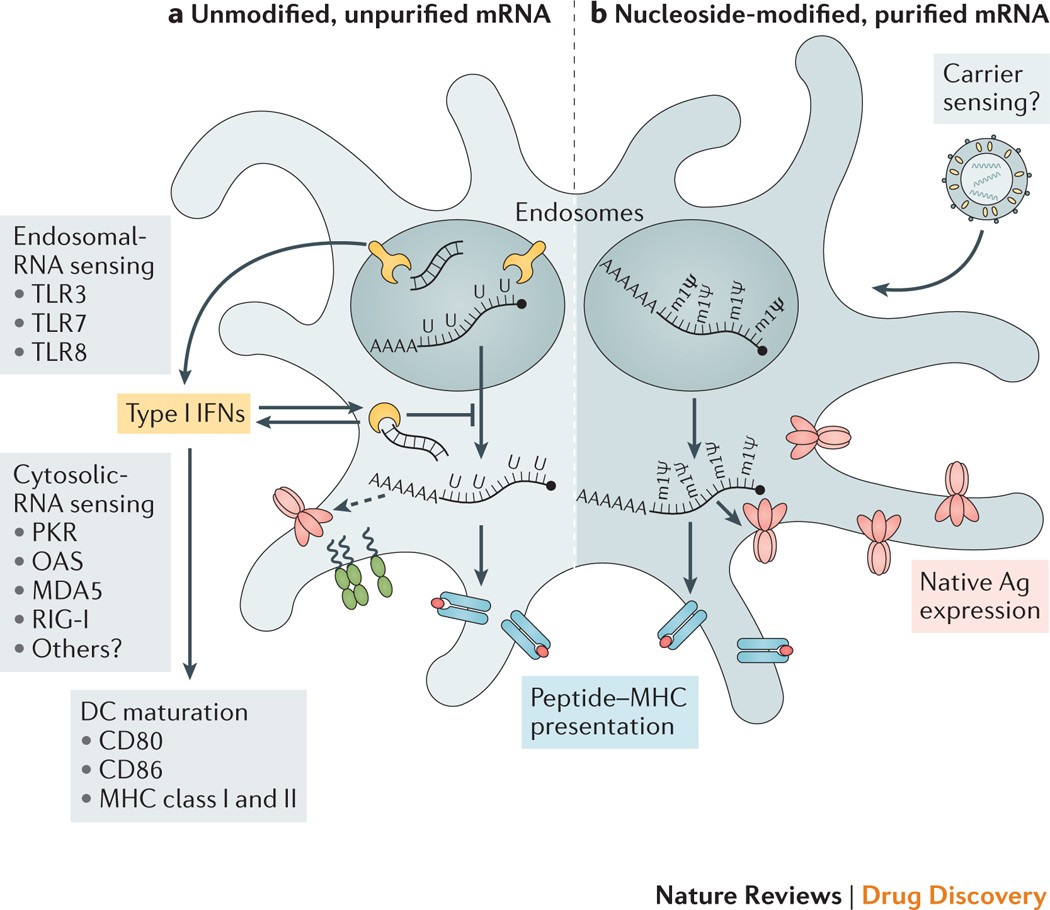
Mrna Vaccines A New Era In Vaccinology Nature Reviews Drug Discovery
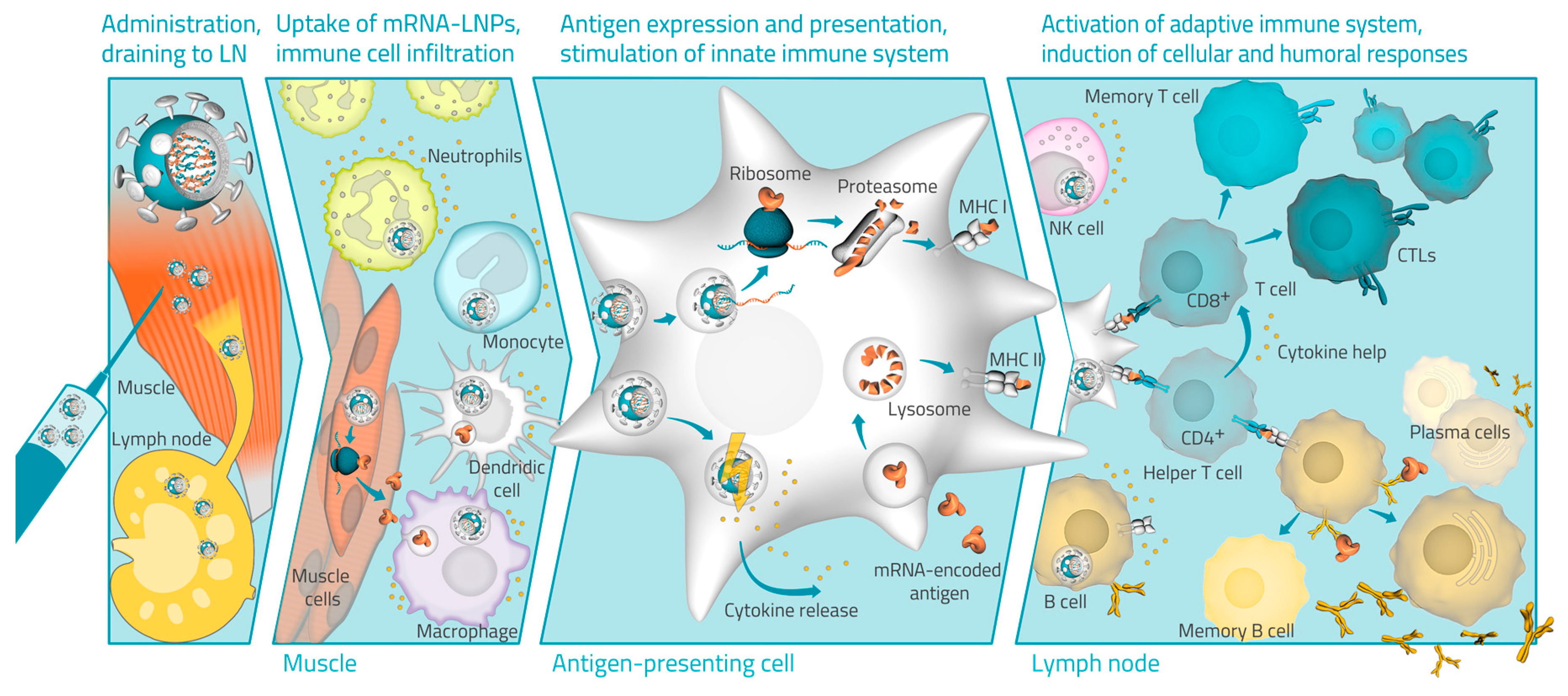
Vaccines Free Full Text Advances In Rna Vaccines For Preventive Indications A Case Study Of A Vaccine Against Rabies Html

Optimization Of Lipid Nanoparticles For Intramuscular Administration Of Mrna Vaccines Molecular Therapy Nucleic Acids
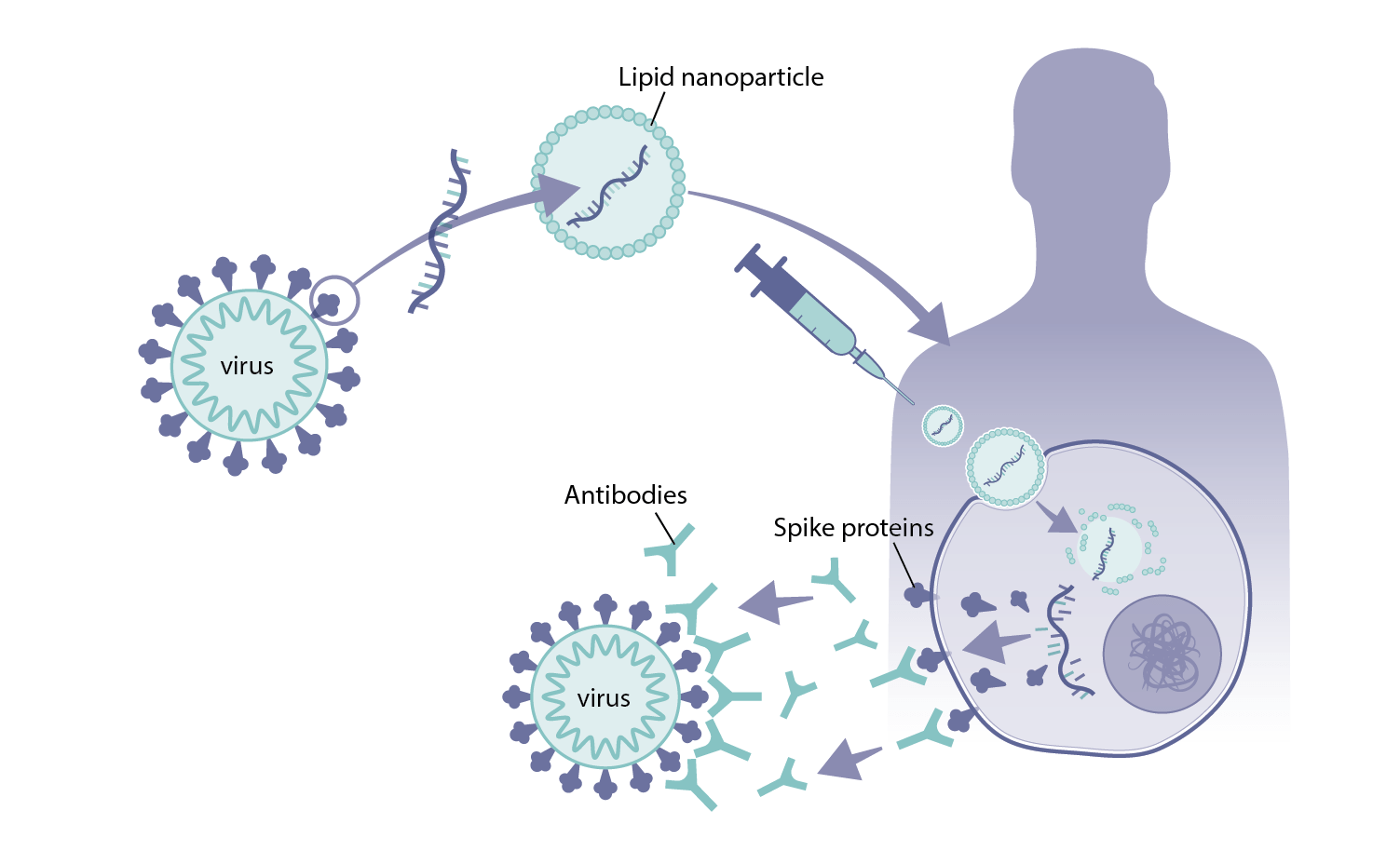
How Were Mrna Vaccines Developed For Covid 19 Health Feedback

Supplemental Materials For Safety And Immunogenicity Of A Mrna Rabies Vaccine In Healthy Adults An Open Label Non Randomised Prospective First In Human Phase 1 Clinical Trial The Lancet
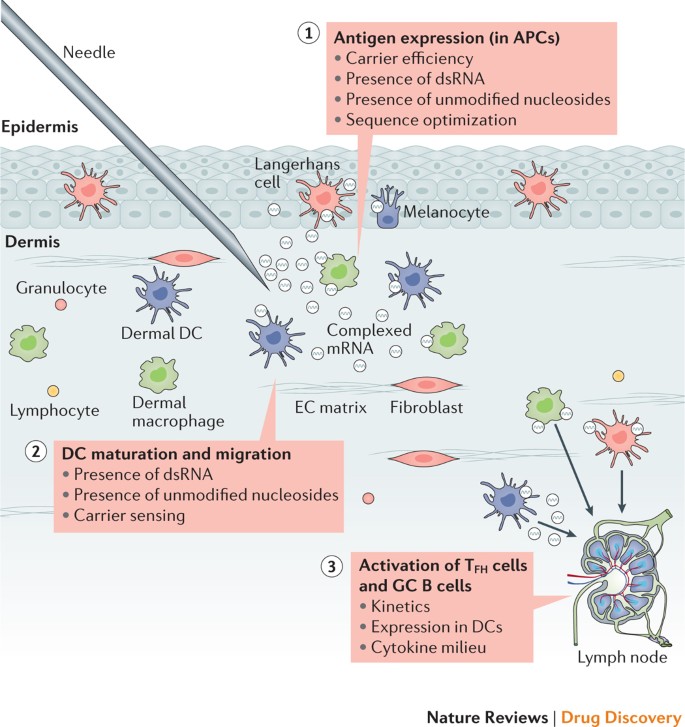
Mrna Vaccines A New Era In Vaccinology Nature Reviews Drug Discovery
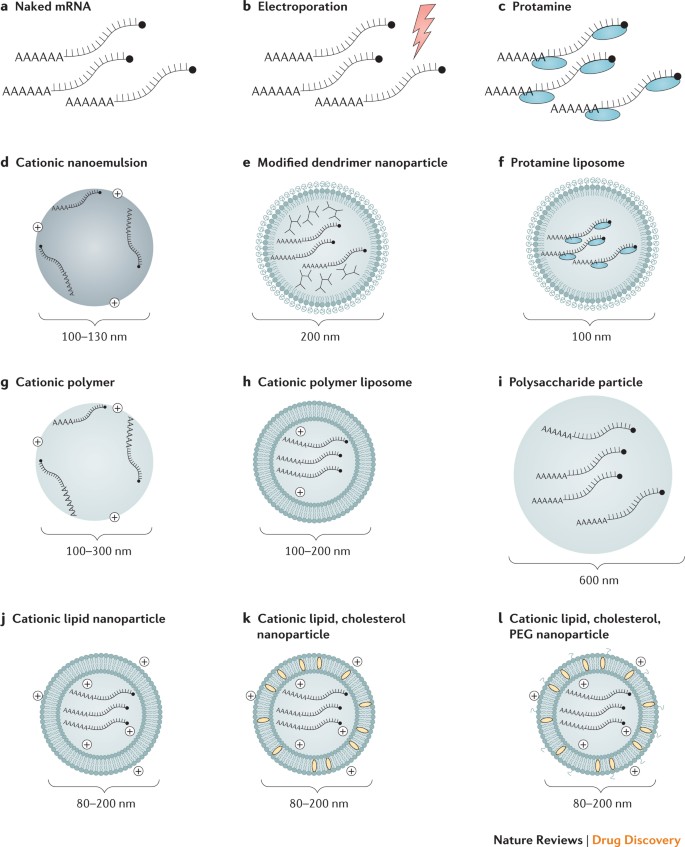
Mrna Vaccines A New Era In Vaccinology Nature Reviews Drug Discovery


Post a Comment for "Mrna Vaccine Safety In Animals"